how many valence electrons does bromine have|Bromine Valence Electrons (And How t : iloilo The electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence electrons of bromine are seven. The elements that have 5, 6, or 7 electrons in the .
Ontario (ON) Lotto Max latest winning numbers, plus current jackpot prize amounts, drawing schedule and past lottery results.
PH0 · What Are Valence Electrons? Definition and Periodic
PH1 · Valence Electrons Chart for All Elements
PH2 · How to Find the Valence Electrons for Bromine (Br)?
PH3 · How to Find the Valence Electrons for Bromine (Br)
PH4 · How to Find the Valence Electrons for B
PH5 · How many valence electrons does bromine have?
PH6 · How many valence electrons does brom
PH7 · How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bro
PH8 · How Many Valence Electrons Does Bromine (Br) Have?
PH9 · How Many Valence Electrons Does Bromine (Br)
PH10 · How Many Valence Electrons Does Bro
PH11 · Determine valence electrons using the periodic table
PH12 · Complete Electron Configuration for Bromine (Br, Br
PH13 · Bromine Valence Electrons (And How t
PH14 · Bromine Valence Electrons
PH15 · Bromine
DU 7 College Admission Result 2024, Subject Choice Form Fill Up know 7 College Result and all college Subject vacant list. https://collegeadmission.eis.du.ac.bd. Dhaka University Affiliated 7 College result can be checked from here. You will login with your SSC and HSC roll number to see your individual result. To see the merit list, you .
how many valence electrons does bromine have*******There are two ways to find the number of valence electrons in Bromine (Br). The first is to use the Periodic Table to figure out how many electrons Bromine h. Mar 23, 2023 Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. The valence electrons of bromine are seven and the valency is one, based on its .Learn how to determine the number of valence electrons for an element using the periodic table. Bromine is in group 17 and has seven valence electrons. See examples, .Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full . The electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence electrons of bromine are seven. The elements that have 5, 6, or 7 electrons in the .
Bromine Valence Electrons (And How t How many valence electrons does Bromine have? Bromine belongs to the group of halogen and hence has similar properties. Being the halogen element Bromine can easily evaporate . Learn what valence electrons are and how to find them for any element. Bromine has seven valence electrons in the 4s and 4p subshells. How many valence electrons does Bromine have? Bromine belongs to the group of halogen and hence has similar properties. Being the halogen element Bromine can easily evaporate .Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan.how many valence electrons does bromine have The highest principal quantum number is 2. There are 2 electrons in the 2s subshell and 2 electrons in the 2 p subshell, giving carbon a total of four valence electrons. Bromine’s ground state .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
The atomic number of Bromine Br is 35. The electronic configuration of Bromine Br can be written as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5; The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a total of seven valence electrons. Therefore, the valence .Solution. Verified by Toppr. Valence electrons found in the s and p orbitals of the highest energy. Bromine has an electron configuration of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 the valence electrons are in the 4 s and 4 p . Bromine is the 35th element of the periodic table so its atomic number is 35. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a bromine atom has thirty-five protons and thirty-five electrons. The number of neutrons in an atom can be determined by the difference between the atomic . This table of element valences includes the maximum valence and most common valence values in chemistry. . (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Remember an element's electron cloud will become more stable by filling, emptying, or half-filling the .
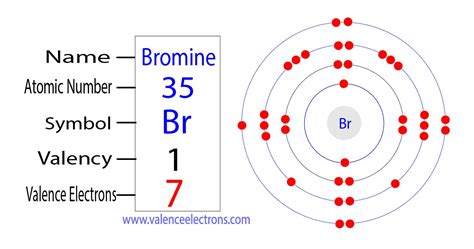
Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising agent, reacting with many elements in order to complete its outer shell.

View this answer. A bromine (Br) atom has 7 valence electrons. Valence electrons are the outermost electrons in an atom, which affect how atoms might react with one. See full answer below.3rd Edition • ISBN: 9781119316152 (17 more) David Klein. 3,105 solutions. Find step-by-step Chemistry solutions and your answer to the following textbook question: How many valence electrons does bromine (Br, atomic no. = 35) have?. Beryllium has two valence electrons. How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period, until the last element is .
For this, bromine difluoride can accept or donate an electron. BrF 2+ will have twenty valence electrons while bromine difluoride donates one electron. Mathematical Analysis: BrF 2+. = 7 + (7×2) – 1. = 20. On the other hand, if bromine difluoride accepts one electron, the valence electrons of BrF 2– will be twenty-two.
Q: How many valence electrons does bromine possess? How many valence electrons does bromine possess? Flexi Says: Bromine (Br) is a halogen and has an atomic number of 35. It has electronic configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Bromine has 7 valence electrons and 4 energy levels. Discuss further with Flexi.How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. B: 1s 2 2s 2 2p 1 (there are three electrons on the highest occupied energy level n=2) In fact, the number of valence electrons goes up by one for each step across a . The \(1s\) electrons in oxygen do not participate in bonding (i.e., chemistry) and are called core electrons. The valence electrons (i.e., the \(2s^22p^4\) part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation \(\ref{3}\)), the electrons in the argon-like closed shell are the core . Bromine - Bromine has an atomic number of 35 with a symbol of Br. It was first discovered in 1826. In its elemental form, it is the diatomic molecule Br 2. At room temperature, bromine is a reddish- brown liquid. Its oxidation states vary from -1, +1, 3, 4 and 5. Bromine is more reactive than iodine, but not as reactive as chlorine.
Play the best and newest free slots for fun in demo mode. Enjoy 16,000+ free demo slots on Casino Guru. Free online slot machines & other games.
how many valence electrons does bromine have|Bromine Valence Electrons (And How t